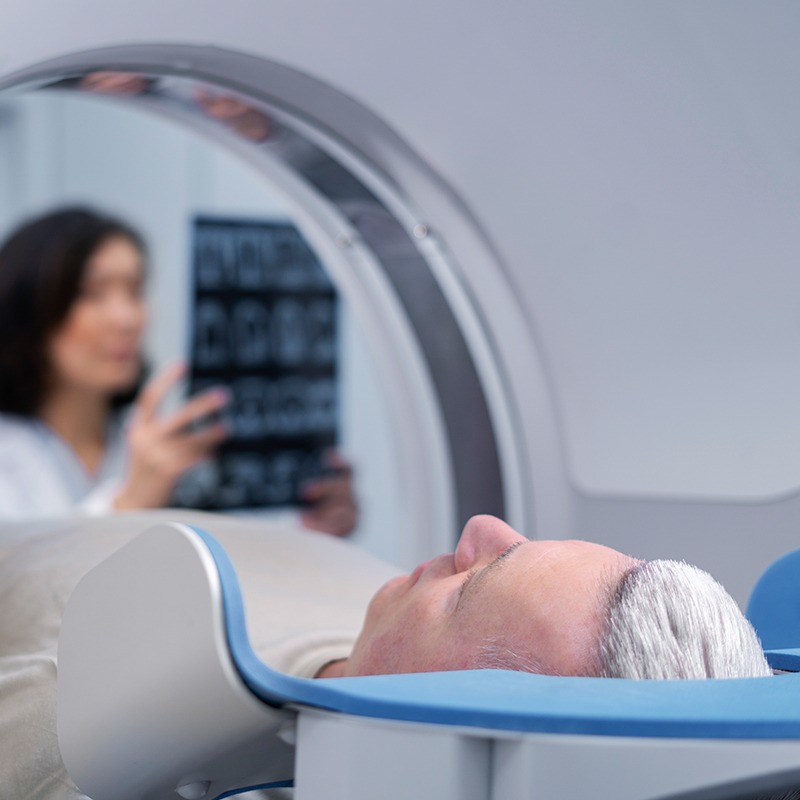
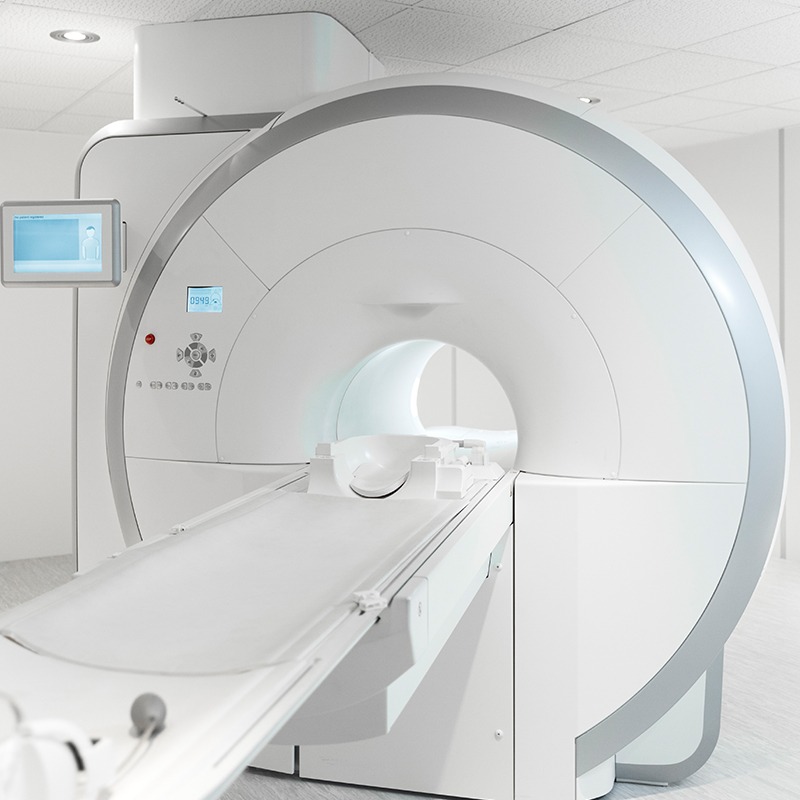
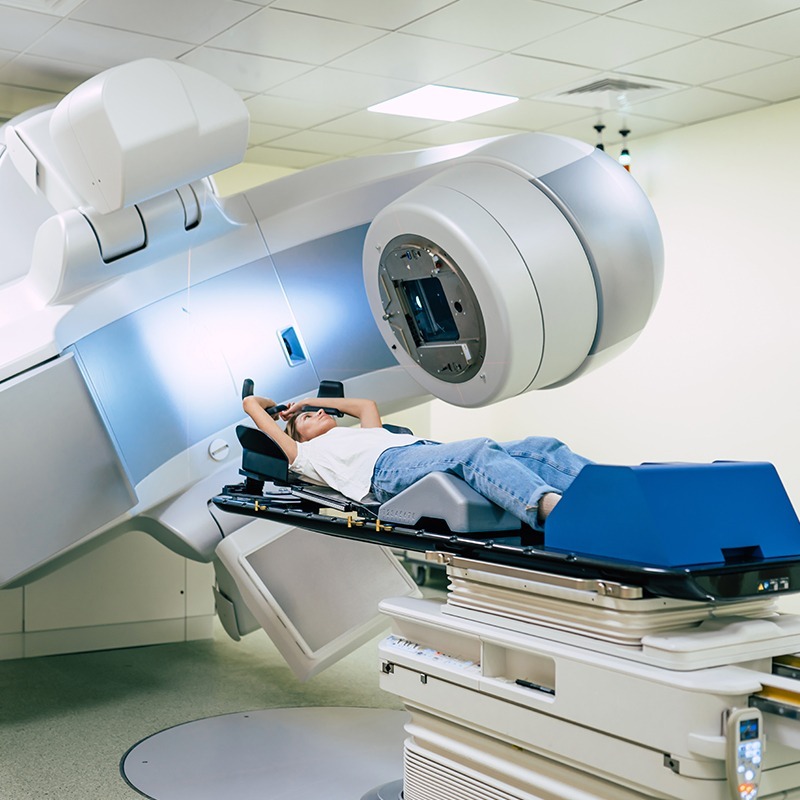
softgate has been developing software for medical devices since the company was founded. The complex and safety-critical processes in the medical field demand maximum software reliability and functionality. To guarantee this, softgate’s development of safety-related software in medical technology is based on EN 62304:2009. Combined with agile software development, we ensure the best possible outcome for your needs.
Safety software for medical technology – our projects’ focal areas:
Diagnostics using high-resolution images and moving system components
Heavy-lift robots in close proximity to patients for diagnostic and therapeutic procedures
Our range of medical software development services
Our highly qualified developers with years of project experience in the field of embedded software development can meet any challenge where there is no standard solution. We will support you every step of the way, from product inception to product development, through to product discontinuation once you have achieved your project goals.
Software development by our project teams based on customized requirements is always done in compliance with current standards and regulations.
Benefits
- Project experience in embedded software development
- Development in compliance with current standards and regulations
- Meets high quality management requirements
- Complete development process from a single source
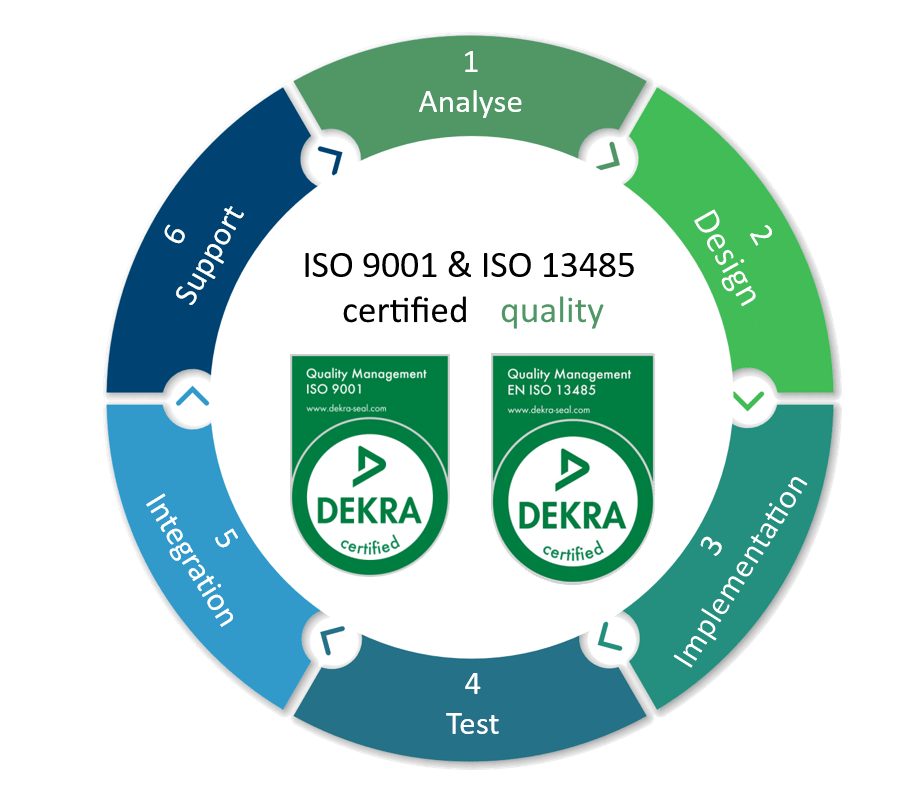
softgate covers the entire development process
- Product lifecycle management/application lifecycle management
- Specifications
- Requirements engineering
- Risk analysis
- Model-based development
- Architecture and design
- Testing on all levels of the V-model
- Code review, unit testing, module testing, integration system, system and acceptance testing
We focus particularly on automatable testing. For requirements tracking, we use an approach that makes it possible to link the document type of the respective development stage to other levels both horizontally and vertically.
What we have achieved for our customers
Integration of operating table and risk analysis
Safety-critical software for an X-ray system
Contact us:
Please feel free to reach out to my team and me with your project requirements. We would be happy to give you an initial overview of how our experienced developers can meet your individual goals.